
 |
LABORATORY OF MOLECULAR GENETICS FOR REPRODUCTION |
| Associate Professor: | TANAKA, Minoru |
| Postdoctoral Fellow: | SAITO, Daisuke |
| Graduate Students: | AOKI, Yumiko 1) |
| NAKAMURA, Shuhei 1) | |
| ARITA, Kaori 1) | |
| KUROKAWA, Hiromi 1) | |
| 1)Graduate School of Hokkaido University | |
|
Sexually dimorphic gonads mainly consist of two different cell lineages, somatic gonadal cells and germ cells. During the course of development, the gonadal precursor cells should be specified through the process of mesoderm patterning, which subsequently associates with primordial germ cells to form the indifferent gonad. Once the indifferent gonad is observed in the gonadal area, sex determination gene is expressed in the gonadal mesoderm and the organogenesis of gonads (sex differentiation) starts in mice. Since sex determination gene of mice was identified in 1990, research has intensively been focused on the testis development. However, for instance, the differentiation of the precursor cells determining sex, the interaction of somatic cells and germline, the differentiation of germline to germ cells having the capability to undergo meiosis, and so on, there remain many important and facinating problems to be solved. Our laboratory aims to reveal the molecular mechanims regarding to the formation of gonads, especially emphasizing on visualizing specific cell lineages in medaka living embryos and by applying moelcular genetics to medaka embryos. |
I. Monitering the formation of gonadsthrough fluorescent germline. |
|
One of the advantages to use medaka is that embryos are transparent and can be seen from outside. This encouraged us to develop transgenic medaka exhibiting GFP exclusively in germline and succesfully established the trangenic lines (olvas -transgenic medaka). During the establishment, we found that two genomic regions, 5 &rsquo promoter region and 3 &rsquo UTR region, are necessary for proper expression of germline-specific gene, olvas(medaka vasa homologue). Furthermore we identified an element, GSE (germline-specific element), in the 3 &rsquo UTR, which mediates both maintenance of RNA exclusively in germline and activation of translation through enhancement of poly(A) elongation. Both mechanisms act independently on GSE and consequently bring about efficient translation in germline, Injection of in vitro synthesized GFP RNA with GSE at its 3 &rsquo end allows to monitor more clearly and brightly the development of germline from early embryogenesis. Bright fluorescence enables us to identify primordial germ cells easily and isolate ESTs that express in germline. We continue to analyze the ESTs currently. |
II. Germline DevelopmentPrimordial germ cells have been characterized as the large cells having electron-dense amorphas structure called germ granules (also called germplasm or nuage). We isolated several ESTs whose products are components of germ granules and revealed that 3 &rsquo UTRs of some ESTs possess cis-element functionally similar to olvas GSE. We synthesized RNA of EST-GFP-3 &rsquo UTR fusion genes and injected it into fertilized eggs. The GFP-fused products were localized in germ granules as has been detected by antibodies. |
|
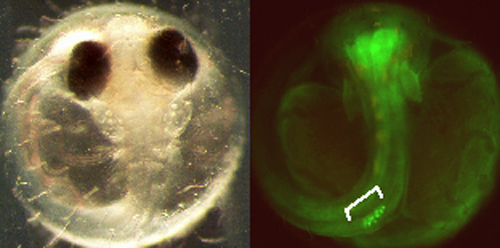
Fig.1 GSE in 3 &rsquo UTR functions to translate RNA exclusively in germline. The picture below right shows fluorescent germline in a gonad. |
 |
|
By keeping track of the structure of the granules with a laser confocal microscopy, we could outline germline development and revealed several developmental stages of germline. Firstly, germline separates from somatic cell lineages at morula stage and is suggested to be fated to primordial germ cells as epiboly movement proceeds at early gastrulation stage. The primordial germ cells near the germ ring move to anterior lateral plate mesoderm and then migrate posteriorly along the embryonic body. Localization of the components on the germ granules differs from that found in the germ cells of the indifferent gonads, suggesting that an additional step is required to differentiate into germ cells which has the capability to undergo meiosis. |
III. Origin of gonadal mesoderm |
|
Many focus have been concetrated on the mechanisms of differentiation into male gonadal mesoderm after sex detemination gene is activated. However, the origin and the lineages of the gonadal precursor cells have remained to be elucidated. As described in section II, germ cells migrate into a gonadal region. This suggests that cells producing guidance substance for primordial germ cells become the precursor of gonadal mesoderm. We constructed a fate map showing where the precursor of gonadal mesoderm is derived using uncaging method. The result indicates that most posterior portions of lateral plate mesoderm converge medially toward an embryonic body to locate bilateral regions where migrating primordial germ cells target. These regions correspond to posterior ends in expression domain of guidance substance. After primordial germ cells meet the precursor cells of gonads in the lateral plate mesoderm, the precursor cells and primordial germ cells move dorsally around a developing hindgut, and finally reach the region where the indifferent gonad will form. It has been suggested by the analysis of mice mutant possessing few germ cells that somatic precursors autonomouly develop to gonadal mesoderm. It is, however, likely that in the process of organogenesis gonads should interact between somatic mesoderm and germ cells. To challenge this problem with our skills and biological backgrounds, we are generating medaka without germ cells by ablation of fluorescent germ cells with a laser or disruption of function of guidance substance. |
IV. Analyses of mutant medaka affecting formation of gonad |
|
Medaka is a small vertebrate and produces next generations in 3 months after hatching. This characteristics are suitable for applying molecular genetics to this small animal. In collaboration with ERATO Kondoh differentiation signaling project, we have been screening mutants affecting primordial germ cells and the formation of gonads. The defects were screended by expression of olvas gene in germ cells. The screening told us the presence, number and distribution pattern of germline at a somitogenesis stage and at ten days post hatching (10 dph). Nine mutants (19 alleles) and twelve mutants (14 alleles) were identified for primordial germ cells and gonads, respectively. The mutants affecting formation of gonads fall into four groups according to their phenotypes. 1) mutants having overproliferating germ cells, 2) mutants with reduced number of germ cells, 3) mutants with fragmented gonads and 4) mutants with scattered germ cells in the gonadal region. We are now characterizing mutant phenotypes and the modes of inheritance. One mutant, totoro, is very interesting in that the similar phenotype has not been described before in other animals. totoro has a swelled large abdomen filled with a gonad. Our analyses show that the phenotype is semidominantly inherited. Gonads are sex-reversed in genetic males and fullfilled with numerous oocytes regardless of its genetic sex. The positional cloning is ongoning with ERATO project and the characterization of the phenotype is also in progress. |
|
Fig.2 sex reversed totoro mutant showing a large abdomen, which is occupied by a gonad with numerous number of oocytes |
 |
|
Another mutant, zenzai, is in good contrast with totoro mutant and is unique in that germ cells are not maintained in the gonad. The mode of inheritance of the phenotype indicates that the allele is recessive. The characterization and the positional cloning are also in progress. |
Publication lists |
|
Mitani, H., Shima, A., Naruse, K. and Tanaka, M. (2004) Medaka genome mapping for functional genomics. In Fish Development and Genetics. pp.612-636. (eds: Gong, Z and Korzh, V) World Scientific. Sasado, T., Morinaga, C., Niwa, K., Shinomiya, A., Yasuoka, A., Suwa, H., Hirose, Y., Yoda, H., Henrich, T., Deguchi, T., Iwanami, N., Watanabe, T., Kunimatsu, S., Osakada, M., Okamoto, Y., Kota, Y., Yamanaka, T., Tanaka, M., Kondoh H. and Furutani-Seiki, M. Mutations affecting early distribution of primordial germ cells in Medaka (Oryzias latipes) embryo. Mech. Dev. (2004) 121, 817-828. Morinaga, C., Tomonaga, T., Sasado, T., Suwa, H., Niwa, K., Yasuoka, A., Henrich, T., Watanabe, T., Deguchi, T., Yoda, H., Hirose, Y., Iwanami, N., Kunimatsu, S., Okamoto, Y., Yamanaka, T., Shinomiya, A., Tanaka, M., Kondoh, H. and Furutani-Seiki. M. Mutations affecting gonadal development in Medaka, Oryzias latipes. Mech. Dev. (2004) 121, 829-839. Naruse, K., Tanaka. M., Mita, K., Shima, A., Postlethwait, J., and Mitani, H. A Medaka Gene Map: The Trace of Ancestral Vertebrate Proto-chromosomes Revealed by Comparative Gene Mapping. Genome Res. (2004) 14, 820-824. Suzuki, A., Tanaka, M., Shibata, N. and Nagahama, Y. Expression of aromatase mRNA and effects of aromatase inhibitor during ovarian development in the medaka, Oryzias latipes. J. Exp. Zool. (2004) 301, 266-273. |
