
 |
DIVISION OF MOLECULAR AND DEVELOPMENTAL BIOLOGY |
| Professor: | TAKADA, Shinji |
| Research Associate: | KOSHIDA, Sumito |
| Technical Staff: | UTSUMI, Hideko |
| NIBB Research Fellow: | KAWAMURA, Akinori |
| JSPS Postdoctoral Fellow: | OHBAYASHI, Norihiko |
| Postdoctoral Fellows: | KURATA, Tomoko |
| AKANUMA, Takashi | |
| TAKADA, Ritsuko | |
| Graduate Student: | YAMAGUCHI, Yoshifumi 1) |
| Technical Assistants: | OHBAYASHI, Akiko |
| ODA, Ritsuko | |
| TAKASHIRO, Kayoko | |
| Secretary: | ODA, Keiko |
| 1) Graduate School of Biostudies, Kyoto University | |
|
One of the research interests of this laboratory is to understand molecular mechanism how a cell signaling molecule, including members of Wnt, BMP and FGF families, regulates different developmental events. A number of evidence indicated that each signal is involved in many aspects of the vertebrate development. For instance, we have revealed that Wnt-3a, a members of Wnt family, plays essential roles in a number of aspects of the mouse development, including somite development, neural crest formation and neural development. However, cellular and molecular mechanisms how a cell signaling molecule regulates these different events. Thus, we are focusing on precise functional analysis of cell-to-cell signals and identification of target genes induced by these signals. Another interest is to understand molecular mechanism of development of the vertebrate trunk, especially somite. One of interesting features of the vertebrate trunk development is that it proceeds gradually. To understand how metameric structures of somites are gradually generated in an anterior to posterior order along the both sides of embryonic body axis and how each somite are characterized differently along the anterio-to-posterior axis, we are also trying genetical approach with the zebrafish. |
I Functional Analysis of molecular targets of Wnt signaling during development |
|
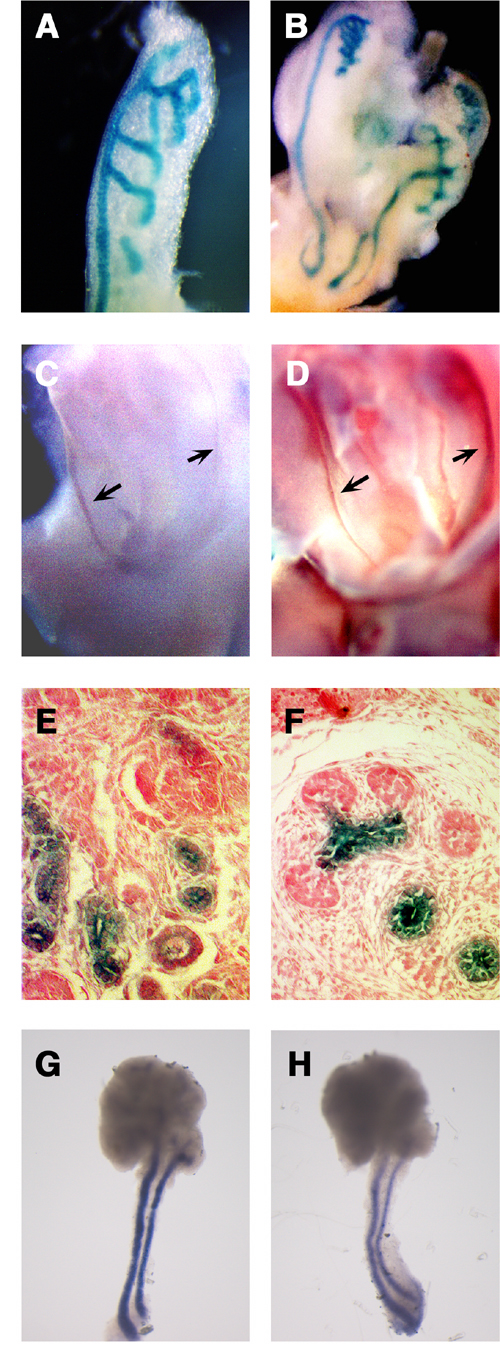
Wnt signaling plays important roles in many aspects of vertebrate development. For revealing the molecular mechanisms underlying each developmental event triggered by Wnt signaling, identification and functional analysis of genes activated or repressed by this signal are important. However, only a few systematic approaches have thus far been reported for identification and functional analysis of Wnt-responsive genes in vertebrate development. The gene-trap methodology is a powerful strategy for the systematic identification and functional analysis of genes in the post genomic era, because this methodology offers the identification of a novel gene, the analysis of its expression pattern, and the generation of its functional mutation in a single experimental approach as described below. In this methodology, random insertion of a gene-trap vector leads to the tagging, and frequently to the disruption, of genes across the genome. Therefore, if ES cells are used for the generation of insertion events, embryonic and adult whole bodies containing tagged and disrupted alleles can be produced. Such an insertion event affords the following advantages for identification and functional analysis of a trapped gene. 1) Nucleotide sequences of a trapped transcript and an insertion site can be determined by 5 ‘ rapid amplification of cDNA ends (RACE) and plasmid rescue method, respectively. The process of identification of a trapped gene has been substantially eased by the recent completion of the genome database. 2) The expression pattern of a trapped gene during development can be easily monitored by the expression of protein tag derived from a gene-trap vector in embryos generated from trapped ES cells. 3) An insertion event has the potential to be mutagenic. Because of these strong advantages, the gene trap methodology has been applied for several studies, including large-scale insertional mutagenesis programs. Although the gene trap methodology basically involves the random integration of a gene-trap vector, this strategy has also been expected to be available for identification and characterization of genes regulated by particular signals. In a modified gene-trap strategy, called induction gene trap, genes are selected by their response to specific secreted signal proteins, including BMP2, activin, nodal, and FGF, added to the culture of gene-trapped cells. Although the in vivo correlation between selected genes and signals has remained unclear in these cases, in vitro prescreening of trapped ES cell lines with secreted signal proteins would be effective for enrichment of genes regulated by specific signals even in normal development. Here, we examined whether gene trap methodology, which would be available for systematic identification and functional analysis of genes, is effective for screening of genes responsive to Wnt signaling during mouse development. We screened out 2 individual clones (Clone 43 and Clone 5) among 794 gene-trapped ES cell lines by their in vitro response to WNT-3A proteins. To examine whether the in vivo expression of these trapped genes was actually regulated by Wnt signal, we examined their expression precisely in mouse embryos. Expression of Clone 43 was temporarily observed in the inner cell mass of blastocysts. Later, Clone 43 was specifically expressed in ductal structures in the mid-gestation stages. This gene was expressed during a number of aspects of the ductal morphogenesis in kidney development. The urogenital expression of Clone 43 was observed first in the nephric duct and mesonephric tubules at E10.5 (Figure 1A) and later in the Wolffian duct, the ureter, the collecting duct, and the distal portion of tubules connected to the collecting duct in the metanephros (Figure 1B, C, E). The expression was restricted to the epithelium, i.e., was not found in the mesenchyme, in these organs (Fig. 1E, F). Clone 43 was also expressed in the ductal epithelium of the salivary glands (submandibular, sublingual, and parotid glands) at E 15.5 (Fig. 1F, G). |
 |
|
Figure 1 Expression of Clone 43 in the ductal development. (A, B) During urogenital development, lacZ reporter was expressed first in the nephric duct and mesonephric tubules at E10.5 (A). At E13, lacZ reporter of clone 43 was also expressed in the Wolffian duct, mesonephric tubules, the ureter, and the collecting duct (B). (C, D) Clone 43 (C) was co-expressed withWnt-7b (D) in the Wolffian duct. (E, F) Clone 43 is expressed in the epithelial cells in the developing kidney (E) and SMG (F). (G, H) Expression of CRTR-1 mRNA (G) and Wnt-5b (H) in the SMG and SLG at E13.5. |
|
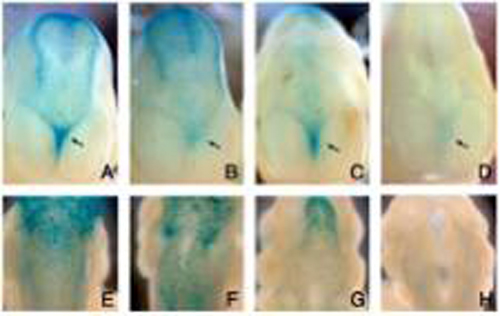
Interestingly, the expression of several Wnt genes was also observed in the ductal structures in which Clone 43 was expressed. In the urogenital development, Wnt-7b, which has been reported to be expressed in the Wolffian duct, the ureter, and the collecting duct at E13.5, was co-expressed with this trapped gene (Figure 1C, D). On the other hand, in the submandibular gland (SMG), the expression of a number of Wnt genes, including Wnt-2, 2b, 3, 4, 5b, 6, 10b, 14, 16, was detected by RT-PCR analysis. Among these Wnt genes, Wnt-5b exhibited an expression pattern spatially and temporally correlated with that of CRTR-1. Wnt-5b was expressed at mid-gestation stages, for instance E13, in the stalks of the SMG and the sublingual gland (SLG), where Clone 43 was expressed (Fig. 1G, H). Thus, the ductal expression of Clone 43 was well correlated with the expression of Wnt-7b and Wnt-5b in the kidney and the salivary gland, respectively The expression pattern of Clone 5, also suggested its close correlation with that of Wnt genes. Expression of Clone 5 was first observed at E 8.5 in rhombomere 5. At E9.5, the expression of this gene was still detected in rhombomere 5 although it was relatively dispersed. Rostral to rhombomere 5, the lacZ-positive cells straggled in the superior membrane of the rhombencephalon. At E10.5-11.5 the expression was observed with strong intensity in scattered neural crest cells over the dorsal diencephalon and mensencephalon, as well as in relatively weak intensity over the dorsal spinal cord. Interestingly, these scattered signals were strong along the dorsal midline adjacent to the roof plate where Wnt-1 and Wnt-3a were expressed. Clone 5 gene was also expressed in mesenchyme in the telencephalic flexure. This mesenchymal expression represents another example of correlation between this gene and Wnt, since Wnt-3a is expressed in the neuroepithelium, adjacent to the mesenchymal cells, in the telencephalic flexure. The expression of Clone 5 gene was also observed in the meninx at E13.5. Taken together, the close correlation between gene expression of the 2 trapped genes and that of several Wnt genes strongly suggests that the trapped genes screened by their in vitro response to WNT proteins were also responsive to Wnt signals in vivo. Furthermore, the expression of these 2 genes was significantly decreased in embryos deficient for several Wnts or in cultures of embryonic tissues treated with a Wnt signal inhibitor. For instance, the positive LacZ signal of Clone 5 gene was absent in neural crest cells migrating from the hindbrain and the spinal cord in the Wnt-1/Wnt-3a double mutant (Figure 2H). In contrast, the lacZ signal was present in the wild-type and in Wnt-1 and Wnt-3a single mutant embryos (Figure 2E-G). These results strongly suggest that Wnt-1 and Wnt-3a were redundantly required for the expression of the clone 5 trapped gene in this region. In addition, the X-gal staining in the mesenchymal cells in the telencephalic flexure was significantly reduced in the Wnt-3a null mutant, as well as in Wnt-1/Wnt-3a double mutant, compared with that in the wild-type and Wnt-1 single mutant embryos (Figure 2A-D). |
|
To examine whether the trapped genes were required for proper development of cells or organs where they were expressed, we generated homozygous mutants for the trapped genes by crossing heterozygous males and females. In homozygotes for the Clone 43 trapped allele, the expression of this gene was almost completely diminished, whereas the expression of a truncated transcript fused to the β -geo gene in the trap vector was strongly detected. Interestingly, 70% of homozygotes for the Clone 43 trapped allele died before 5 weeks after birth. Most of in the Clone 43 homozygous mice exhibited hypoplasia of the kidney at postnatal day30, whereas the heterozygous and wild-type mice in the same litters showed no obvious defect in their kidney. Thus, Clone 43 is likely to be required for proper development of the kidney, in which this gene is expressed. On the other hand, no obvious defect has yet been observed in homozygotes for the clone 5 trapped allele. Thus, the in vivo expression of the 2 trapped genes, which were screened by their in vitro response to Wnt, was also dependent on the Wnt activity. Furthermore, homozygotes for a trapped allele showed a morphological phenotype in the kidney, where the trapped gene was expressed overlappingly with Wnt-7b. These results indicate that an inductive gene trap in ES cells is likely to be effective for screening and functional analysis of genes induced by Wnt signaling during embryogenesis. |
 |
|
Figure 2 Down-regulation of the expression of Clone 5 gene in Wnt-3a and Wnt-1/Wnt-3a mutants. (A-D) Frontal views of the expression of Clone 5 in the telencephalic flexure (arrow) at E11.5. (E-H) Dorsal views of the expression of Clone 5 gene in the hindbrain at E11.5. Wild type (A, E), Wnt-3a null mutant (B, F), Wnt-1 mutant (C, G), Wnt-1 and Wnt-3a double-null mutant (D, H). |
II Genetical approaches for revealing molecular mechanism of trunk development in zebrafish |
|
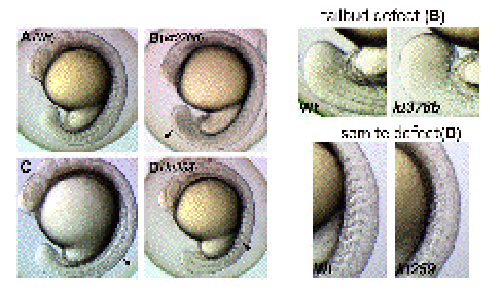
To identify genes involved in several aspects during early embryogenesis of the vertebrate, we have performed screening of zebrafish mutants induced by ENU, a chemical mutagen. Until now, we have screened 630 of F2 families and found a number of mutants whose phenotypes are different from those already reported. For instance, some of these mutants displayed defects in the somite and tailbud development (Figure 3). Cloning of genes that are responsible for these defects is in progress. To complement a forward genetical approach, we have also screened genes expressed in the tailbud and presomitic mesoderm, in which somite progenitors exist. Until now, we have identified 50 genes that are expressed preferentially in these regions. To examine developmental roles of these genes, functional analysis of these genes has been performed by injecting morpholino anti-sense oligonucleotides. |
 |
|
Figure 3 Zebrafish mutant embryos which display abnormal shape of the tailbud (kt378b) and a segmentation defect of somites (kt259). |
Publication List: |
|
Hasegawa H, Ashigaki S, Takamatsu M, Suzuki-Migishima R, Ohbayashi N, Itoh N, Takada S, Tanabe Y. (2004) Laminar patterning in the developing neocortex by temporally coordinated fibroblast growth factor signaling. J Neurosci. 24, 8711-8719. Kamata T, Katsube K, Michikawa M, Yamada M, Takada S, Mizusawa H. (2004) R-spondin, a novel gene with thrombospondin type 1 domain, was expressed in the dorsal neural tube and affected in Wnts mutants. Biochim Biophys Acta. 1676, 51-62 Muroyama Y, Kondoh H, Takada S. (2004) Wnt proteins promote neuronal differentiation in neural stem cell culture. Biochem Biophys Res Commun. 313, 915-921. Naito M, Katayama R, Ishioka T, Suga A, Takubo K, Nanjo M, Hashimoto C, Taira M, Takada S, Takada R, Kitagawa M, Matsuzawa S, Reed JC, Tsuruo T. (2004) Cellular FLIP inhibits beta-catenin ubiquitylation and enhances Wnt signaling. Mol Cell Biol. 24, 8418-8427. Satoh K, Kasai M, Ishidao T, Tago K, Ohwada S, Hasegawa Y, Senda T, Takada S, Nada S, Nakamura T, Akiyama T.(2004) Anteriorization of neural fate by inhibitor of beta-catenin and T cell factor (ICAT), a negative regulator of Wnt signaling. Proc Natl Acad Sci U S A. 101, 8017-8021 Usui H, Shibayama M, Ohbayashi N, Konishi M, Takada S, Itoh N. (2004) Fgf18 is required for embryonic lung alveolar development. Biochem Biophys Res Commun. 322, 887-892. |
