
 |
DIVISION FOR SEX DIFFERENTIATION |
| Professor: | MOROHASHI, Ken-ichirou |
| Research Associates: | FUKUI, Yuko |
| OGAWA, Hidesato | |
| Technical Staff: | OKA, Sanae |
| Postdoctoral Fellows: | TSUCHIYA, Megumi |
| BABA, Takashi 1) | |
| MOHAMAD, Zubair 2) | |
| Graduate Students: | KUSAKA, Masatomo 3) |
| KOMATSU, Tomoko 3) | |
| MATSUYAMA, Makoto 3) | |
| FATCHIYAH 3) | |
| SATO, Yuko 3) | |
| MIYABAYASHI, Kanako 4) | |
| Technical Assistants: | OWAKI, Akiko |
| ISHIKAWA, Azusa | |
| KOIKE, Yukari | |
| GOUNO, Hiroe | |
| TOMIDA, Sanai | |
| Secretary: | SUGIURA, Mio 2) |
| 1) JSPS Postdoctral Fellow | |
| 2) SORST, JST | |
| 3) Graduate University for Advanced Research | |
| 4) Graduate School of Tohoku University | |
|
Although sexual dimorphism manifests most obviously in the gonads (testis and ovary), it can be observed in the other parts of the entire animal body. For instance, it is well known that several tissues such as the external genitalia, muscle, and brain exhibit sexual dimorphisms in terms of their structures and functions. This process of sex differentiation can be divided into three steps. The first occurs at fertilization, during which the sexes of fertilized eggs are determined genetically by combination of sex chromosomes. As the second step, the individuals carrying XY and XX sex chromosomes develop the testis and ovary, respectively, in the case of mammals. Sex differentiation of the gonads usually proceeds during fetal stages. Thereafter, the gonads control the sexes of the whole body through the function of sex steroids synthesized in the sexually differentiated gonads. Therefore, the gonadal sexes are quite important for sex differentiation of the animals. It has been established that a number of transcription factors play crucial roles in the process of gonadal differentiation. Some of these factors, such as SRY, WT1, DAX-1, SOX9 and ARX were identified as the products of genes responsible for human diseases that display structural and functional defects in several tissues including the gonads. The functions of the other transcription factors such as Ad4BP/SF-1, Emx2, M33, and Lhx9 were elucidated by the phenotypes of the gene disrupted mice. In addition, the expression profiles in terms of their gonadal distribution and sexual dimorphism strongly suggested the functional significance at the early stage of gonadal differentiation. However, it remains to be clarified how the transcription factors above regulate their target gene transcription and how the genes encoding the transcription factors are regulated by upstream regulators. Studies from above two directions are quite important to define the gene regulatory cascade and the molecular mechanisms that supports sex differentiation of the gonad. Tentatively, we hypothesize that the sexually indifferent gonads determine their sexes under the control of two opposite signals, one of which is the signal for testicular differentiation and the other is the signal for ovarian differentiation (Fig. 1). Probably the nature of the signals might be a transcriptional activity of a certain transcription factor expressed in the fetal gonads, or otherwise might be activity of a certain type of growth factor. The study in this division has been performed mainly from the aspect of the transcriptional control as the signal for the sex differentiation. As indicated in Fig. 1, a number of transcription factors/DNA binding proteins are expressed in the developing gonads. Based on the symptoms of human patients and the phenotypes of gene knockout mice, Ad4BP/SF-1, Emx2, and Wt1 are indispensable for the gonadal development. With respect to the signals for gonadal sex differentiation, Sry, M33, Arx, Dhh, Sox9, Pdgf, Wt1, and Fgf9 are assumed to act as the testicular signals while Dax-1 and Wnt4 are the ovarian signals. |
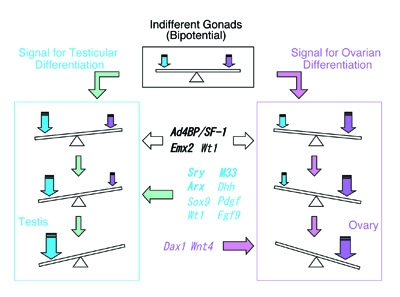 |
Fig. 1, Gondal sexes are determined by balance of signals for testicular and ovarian differentiation. |
I. Functional analyses of VinexinIn order to define the function of Ad4BP/SF-1, we have screened a two-hybrid library prepared form mouse embryonic gonads. Vinexin was one of the clones isolated by the screening. Two isoforms of Vinexin, Vinexin α and β, had previously been identified, however, the clone isolated in this study was distinct from those two isoforms in the 5 amino acid coding region. Thus, this novel isoform was tentatively designated Vinexin γ. Structurally, Vinexin γ is composed of 680 amino acids corresponding to the 57th to 736th amino acids of Vinexin α (76 kD). Analyses of the gene structure indicated that this difference is due to alternative usage of the first exon. In silico screening revealed the presence of Vinexin γ in other animal species; human SCAM-1 (SH3-Containing Adaptor Molecule-1) and rat SCAM-1. In situ hybridization and immunohistochemical studies showed that Vinexin γ started to be expressed in the sexaually indifferent gonads (E11.5), and thereafter expressed specifically in Sertoli cells in the testis (Fig. 2). |
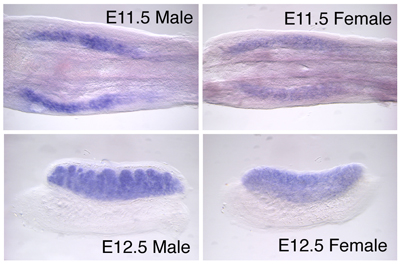 |
Fig. 2, Expression of Vinexin γ in the developing mouse gonads. Vinexin γ is expressed in the sexually indifferent gonads (E11.5) of both sexes (Male and Female). The expression continues at E12.5 gonads. In the male gonad, the expression of Vinexin γ is clearly restricted in Sertoli cell. |
|
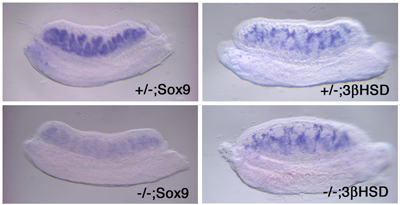
For the function of Vinexin, it has been discussed that the molecule is involved in MAPK signaling through interacting with ERK. Thus, we investigated whether Vinexin γ interacts with the components of MAPK cascade. Interestingly Vinexin γ was found to interact with c-Raf as well as ERK, suggesting that Vinexin γ acts as a scaffold protein. Moreover, our data indicated that Vinexin γ activates MEK and ERK through interaction with c-Raf and ERK. Ultimately in culture cell study, it was revealed that Sox9 transcription was induced by Vinexin γ. This up-regulation of Sox9 expression disappeared when the cells were treated with a specific MEK inhibitor. In order to reveal the function of Vinexin γ during gonadal development, we generated a Vinexin γ-specific gene-disrupted mouse. Unexpectedly, the homozygous mutant mice of both sexes were fertile with morphologically normal gonads. The mice showed decreased Sox9 expression, and the decreased levels varied among individuals. Indeed, even in the most affected individuals, we failed to detect either complete disappearance of Sox9 expression or developmental defects in the XY gonad. Since other MAPKs, JNK and p38, were not activated ectopically in the Vinexin γ-/- fetal XY gonad, it is unlikely that the Vinexin γ-dependent function of ERK was displaced by the other MAPKs. Together, the moderate phenotype displayed by the Vinexin γ-/- gonad suggested that MAPK activation is not critical for testis formation. Consistent with our observations, it was recently reported that a MAP kinase inhibitor did not inhibit testis cord formation in gonadal organ culture. Alternatively, the relatively moderate phenotype displayed by the Vinexin γ-disrupted mice might be due to the following two possibilities. Firstly, Vinexin α and Vinexin β might function redundantly. Indeed, these two isoforms share three tandem repeats of SH3 domains, and moreover, Vinexin β was still evident in the gonad of Vinexin γ-disrupted mice. However, given that Vinexin γ contains its specific N-terminal half and the expression in the fetal testis does not appear to overlap with that of Vinexin β, it is unlikely that the function of Vinexin β is completely redundant with that of Vinexin γ. Secondly, the functions of two closely related proteins, Arg-binding protein 2 (ArgBP2) and c-Cbl-associated protein (CAP/ponsin/SH3P12), should be noted. Structurally, besides the presence of three SH3 domains in common at their C-termini, these proteins share a sorbin domain with Vinexin γ in their N-terminal halves. Moreover, expression of ArgBP2 and CAP/ponsin/SH3P12 was detected in the fetal gonads (E12.5) of both sexes by RT-PCR and whole-mount in situ hybridization, although these signals were weak. Thus, it might be possible that their features functionally and spatially overlapped with Vinexin γ failed to display drastic gonadal defects in the Vinexin γ-disrupted mice. Taken together, Vinexin γ seems to be implicated in regulation of Sox9 gene expression by modulating MAPK cascade in mouse fetal gonads. |
 |
|
Fig. 3, Expression of Sox9 and 3βHSD in Vinexin γ knockout mice. The developing gonads (E12.5) of the knockout (-/-) and heterozygous (+/-) mice were subjected to in situ hybridization probed with Sox9 (Sertoli cell marker gene) and 3β-hydroxysteroid dehydrogenase (3βHSD) (Leydig cell marker gene). Representative decreased expression patters are shown for Sox9 in the knockout. The expression of 3βHSD in Leydig cells seemed not to be affected by Vinexin γ knockout. |
II. Characterization of VMH-specific enhancer of Ad4BP/SF-1 gene |
|
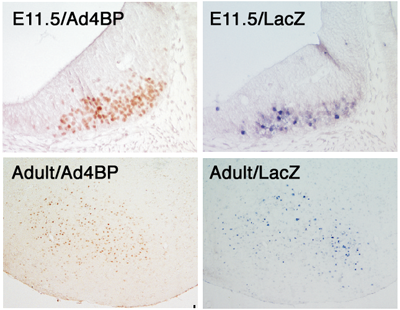
It has been generally accepted that genes are divided into two types according to their expression profiles. Firstly, there are the so-called house-keeping genes with ubiquitous expression among tissues and constitutive expression during developmental processes. In contrast, other genes display tissue-specific and/or developmental stage-specific expression. Many genes have been revealed to contain enhancer sequences, which may explain the molecular basis of regulation of these spatially and temporally restricted-expression. Ad4BP/SF-1 is a transcription factor essential for animal reproduction. Based on the phenotypes observed in gene-disrupted mice, Ad4BP/SF-1 is thought to be involved in establishment of the hypothalamic-pituitary-gonadal axis. However, the mechanisms underlying tissue-specific expression of Ad4BP/SF-1 are largely unknown. We have investigated the cis-regulatory regions of the mouse Ad4BP/SF-1 gene by transgenic mouse assays, and recently we identified a fetal adrenocortical-specific, pituitary-specific, and ventromedial hypothalamic nucleus (VMH)-specific enhancers. In the present study, we identified a VMH-specific enhancer in intron 6 of the Ad4BP/SF-1 gene. Functionally, the enhancer was characterized by driving VMH-specific expression of the lacZ reporter gene when the enhancer was localized with the basal promoter of Ad4BP/SF-1. Moreover, the VMH-specific expression was also reproduced with the hsp68 basal promoter, indicating that the VMH-specific expression can be driven primarily by this enhancer. Structurally, the sequence is conserved among animal species (mouse, human, and chicken), thus strongly indicating that the conserved sequences probably function as a VMH-specific enhancer beyond animal species. |
 |
|
Fig. 4, Comaprison between endogenous Ad4BP/SF-1 and transgene-derived expression of lacZ. Transgene was constructed with a VMH-specific enhancer DNA fragment and lacZ gene as a reporter. Transgenic mice at fetal age (E11.5) and adult were used for the study. The endogenous expression of Ad4BP/SF-1 was examined immunohistochemically. |
|
Further detailed transgenic analyses of the VMH enhancer revealed that the enhancer contains suppressive and activating elements. Mutation of the former element resulted in ectopic lacZ reporter gene expression in an area dorsal to the intrinsic expression domain and in the ventricular zone, while mutations in the latter containing ATTA motifs led to the disappearance of the reporter gene expression, suggesting the involvement of homeobox proteins. Brain regionalization is regulated primarily by the functions of growth factors together with regionally expressed homeobox proteins. Moreover, it has been shown that combined expression of these homeobox proteins is essential to establish progenitor cell identity and neuronal cell subtypes during forebrain patterning. Indeed, several homeobox proteins are known to be expressed in various regions of the developing brain. Among these, it is noteworthy that the expression domain of Nkx2.1 overlaps with that of Ad4BP/SF-1 until E18.5. Nkx2.1 is a member of the Nkx homeoprotein family, and plays important roles in ventral forebrain development. In particular, in Nkx2.1-null mice, the ventromedial and dorsomedial nuclei were underdeveloped and fused in the midline. The expression profile of Nkx2.1 and the phenotypes of the gene-disrupted mice prompted us to analyze the effect of gene disruption on the VMH enhancer function. Disappointingly, however, expression of the lacZ reporter gene driven by the VMH enhancer persisted even in the Nkx2.1 knockout background, suggesting that Nkx2.1 is not essential for the enhancer function. In this context, it is quite interesting to consider the potential redundant function of another family member, Nkx2.4, of which expression is overlapped with Nkx2.1 in the developing forebrain. Meanwhile, considering that the lacZ-positive cells in the ventral diencephalon of the Nkx2.1 knockout mice were fewer in number than in the wild-type from the early developmental stage of VMH, Nkx2.1 could be partially involved in the VMH-specific enhancer function. Through the function of the VMH enhancer, Nkx2.1 might regulate the amount of Ad4BP/SF-1 expressed, and thereby stimulate VMH cell proliferation. Indeed, it was recently reported that cell numbers in adrenocortical primordium are tightly dependent on the amount of Ad4BP/SF-1. |
Publication List: |
|
Hasegawa, T., Fukami, M., Sato, N., Sasaki, G., Fukutani, K., Morohashi, K., Ogata, T. (2004) Testicular Dysgenesis without Adrenal Insufficiency in a 46,XY Patient with a Heterozygous Inactive Mutation of Steroidogenic Factor-1.J. Clin. Endocrinol. Metab. 89, 5930-5935. Komatsu, T., Mizusaki, H., Mukai, T., Ogawa, H., Baba, D., Shirakawa, M., Hatakeyama, S., Nakayama, K., Yamamoto, H., Kikuchi, A., Morohashi, K. (2004) SUMO-1 Modification of the Synergy Control Motif of Ad4BP/SF-1 Regulates Synergistic Transcription between Ad4BP/SF-1 and Sox9. Mol. Endocrinol. 18, 2451-2462. Ogawa, H., Yu, R. T., Haraguchi, T., Hiraoka, Y., Nakatani, Y., Morohashi K., Umesono, K. (2004) Nuclear structure-associated TIF2 interacts with glucocorticoid receptor and its target DNA. Biochem. Biophys. Res Commun. 320, 218-225. Mitsunaga, K., Araki, K., Mizusaki, H., Morohashi, K., Haruna, H., Nakagata, N., Giguere, V., Yamamura, K., Abe K. (2004) Loss of PGC-specific expression of the orphan nuclear receptor ERR-β results in regulation of germ cell number in mouse embryos. Mech of Dev. 121, 237-246. Toda, K., Okada, Y., Zubair, M., Morohashi, K., Saibara, T., Okada T. (2004) Aromatase-knockout mouse carrying an estrogen-inducible enhanced green fluorescent protein gene facilitates detection of estrogen actions in vivo.Endocrinol. , 1880-1888. Kato, M., Petras, D.S., Kitamura, K., K., Morohashi, K., Abuelo, D. N., Barr, M., Bonneau D.,, Brady, A., Carpenter, N. J., Frisone, F., Fukuda, T., Guerrini, R., Iida, E., Itoh, M., Lewanda, A. F., Nanba, Y., Oka, A., Proud, V. K., Russel, K. L., Saugier-Veber, P., Schelley, S.L., Selicorni, A., Shaner, R., Silengo, M., Stewart, F., Sugiyama, N., Toyama J.,, Toutain, A., Vargas, A. L., Yanazawa, M., Zackai E. H., Dobyns W. B. (2004) Mutations of ARX are associated with striking pleiotropy and consistent genotype-phenotype correlation. Human Mutation 23, 147-159. |
