
 |
DIVISION OF REPRODUCTIVE BIOLOGY |
| Professor: | NAGAHAMA, Yoshitaka |
| Associate Professor: | YOSHIKUNI, Michiyasu |
| Research Associates: | OKUBO, Kataaki |
| OHNO, Kaoru | |
| Technical Staff: | KOBAYASHI, Hiroko |
| JSPS Postdoctoral Fellows: | OHTA, Kohei |
| YI, Mei-Sheng | |
| BHANDARI, Ramji | |
| CHAUBE, Radha | |
| Postdoctoral Fellows: | MATSUDA, Masaru 2) |
| IJIRI, Shigeho 1) | |
| SAKAI, Fumie 1) | |
| GUAN, Guijun 1) | |
| SHIBATA, Yasushi 1) | |
| WANG, De-Shou 1) | |
| LAU, En-Lieng | |
| PAUL, Bindhu | |
| Graduate Students: | OHMURO-MATSUYAMA, Yuki 3) |
| KURITA, Kayoko 3) | |
| ZHOU, Lin-Yan 3) | |
| Visiting Scientist: | TANAKA, Masaki |
| Technical Assistants: | HAYAKAWA, Rie 1) |
| UNO, Eri 1) | |
| HARA, Ikuyo 1) | |
| SUGITA, Chiyoko | |
| TAKAKI, Chikako | |
| Secretary: | SHIMADA, Yu 1) |
| 1) CREST, JST; 2) PREST, JST; | |
| 3) Graduate University for Advanced Studies | |
|
Our research focuses on (1) the identification of regulators and steroidal mediators involved in sex determination, gonadal sex differentiation and gametogenesis, and (2) the mechanisms of synthesis and action of these mediators. I. Sex-determining geneMedaka possesses a stable genetic XX/XY sex determining system. Using positional cloning and detailed sequence analysis of BAC clones by shotgun sequencing, we identified DMY (DM domain gene on the Y chromosome) as a strong candidate for the sex-determining gene of medaka. DMY encodes a protein of 267 amino acids including the highly conserved DM domain. The DM domain was named after a related DNA binding motif found in two proteins, dsx and mab-3, involved in sexual development in Drosophila and C. elegans, respectively. Our loss- and gain-of-function studies indicate that DMY is the sex-determining gene of medaka. DMY provides the first example of a sex-determining gene in non-mammalian vertebrates (Fig. 1). A phylogenetic tree based on the amino acid sequence including the DM-domain shows that DMY was derived from DMRT1 immediately before speciation of O. latipes and O. curvinotus.
DMY mRNA and protein are expressed specifically in the somatic cells surrounding primordial germ cells (PGCs) in the early gonadal primordium, before morphological sex differences are seen. However, somatic cells surrounding PGCs never express DMY during the early migratory period. Expression of DMY persists in Sertoli cells, from PGC-supporting cells to Sertoli cells, indicating that only DMY positive cells enclose PGCs during mitotic arrest after hatching. |
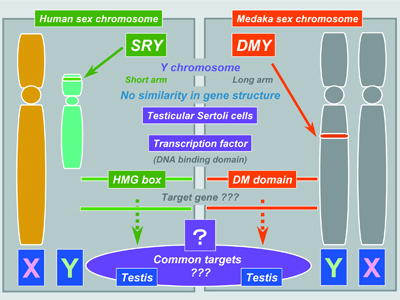 |
Fig. 1 Comparison of two known sex-determining genes in vertebrates, SRY/Sry in mammals and DMY in medaka. |
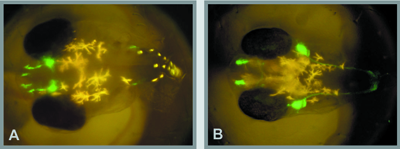
II. Endocrine regulation of gonadal sex differentiationNile tilapia, Oreochromis niloticus, is an excellent example of the precise nature of steroidogenic actions during gonadal sex differentiation. In this fish, all genetic female (XX) or male (XY) broods can be obtained by artificial fertilization of normal eggs (XX) and sex-reversed, pseudo male sperm (XX), or normal eggs (XX) and super male sperm (YY), respectively. In all genetic female tilapia, steroid-producing cells in ovaries prior to and during sex differentiation express all of the steroidogenic enzymes required for estrogen production. Estrogen receptors α and β first appear in XX gonads prior to sex differentiation. The treatment of XX fry with fadrozole (aromatase inhibitor) or tamoxifen (estrogen receptor antagonist) caused complete sex reversal to functional males. These results, together with evidence that the XX sex reversal induced by fadrozole was rescued completely with simultaneous estrogen treatment, suggest that endogenous estrogens are required for ovarian differentiation in tilapia. In contrast to XX fry, steroid-producing cells appear to differentiate after gonadal differentiation in XY fry. The DMRT1 gene is expressed male-specifically in testicular Sertoli cells during sex differentiation. Application of DMRT1 morpholino antisense oligonucleotides (DMRT1 knock down) blocked androgen-induced masculinization of XX gonads. These results suggest an important role of DMRT1 in testicular differentiation in tilapia. To identify the down-stream gene products of estrogen during ovarian differentiation, we have performed subtraction hybridization using mRNA derived from normal and estrogen treated XY gonads of tilapia. Interestingly, one of the up-regulated gene products was aromatase, indicating that endogenous aromatase activity is up-regulated in XY fry after the induction of XY sex reversal by estrogen. It is also of interest that one of the down-regulated gene products was DMRT1, providing evidence to support that DMRT1 may function as a pivotal gene for male gonadal sex differentiation. III. Sex-changing fishThe goby, Trimma okinawae, can serially change sex in either direction. Their gonads contain ovarian and testicular portions simultaneously; however, individuals typically produce one gamete type, change sex and then produce the other type. They do not produce mature sperm and oocytes at the same time. Thus, T. okinawae provides an ideal model for studies on the mechanisms of sex change. Aquarium experiments were carried out in the laboratory. Two males (M+M) or two females (F+F) were kept in separate tanks. In the M+M aquarium, the smaller male changed its sex to female. On the contrary, the larger female changed its sex to male in the F+F aquarium. Behavioral changes occurred within 30 minutes of social manipulation. After pairing, the larger male or female attacked the smaller fish, which fled and often hid in a nest. After 30 min, however, the larger fish began to court the smaller fish. These results suggest that the sex of brain/behavior changes quickly and easily, regardless of gonadal sex. Brain sex of sequential hermaphrodite might be determined independent of gonadal effect and were induced only by social cues. IV. Embryonic development of gonadotropin- releasing hormone neuronsAppropriate development of forebrain gonadotropin- releasing hormone (GnRH) neurons is essential for establishing and maintaining reproductive competence, yet the mechanisms underlying this process are poorly defined. We established transgenic medaka lines that expressed GFP under the control of the gnrh1 and gnrh3 promoters, and they allowed prolonged in vivo imaging of GnRH neuronal behavior at any stage of development in a noninvasive manner (Fig. 2). Our images revealed that preoptic gnrh1 neurons and terminal nerve ganglion gnrh3 neurons originate in the olfactory region, while the medial basal telencephalic gnrh1 neurons arise from the rostral telencephalon. We also found that the diencephalic neuronal population transiently expresses gnrh1 during embryogenesis, that the trigeminal ganglion neurons express gnrh3, and that gnrh3 is maternally expressed in oocytes and embryos. Taken together, the transgenic medaka provides a useful model system for studying GnRH neuronal development including disorders of GnRH deficiency. |
 |
|
Fig. 2 Transgenic medaka embryos that express GFP under the control of gnrh1 (A) and gnrh3 (B) promoters. |
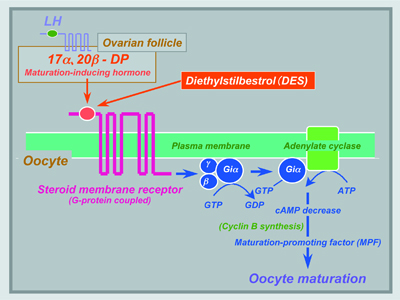
V. Endocrine regulation of spermatogenesisUsing an organ culture system for eel, Anguilla japonica, testes consisting of spermatogonia and inactive somatic cells, we have shown that the hormonal regulation of spermatogenesis in eel testes involves the gonadotropin stimulation of Leydig cells to produce 11-ketotestosterone (11-KT), a potent androgen in fish. In turn, 11-KT activates Sertoli cells to stimulate the production of activin B. To clarify the mechanism of the initiation and progression of spermatogenesis, we examined the expression pattern of A-type cyclins and Dmc1 during gonadotropin-induced spermatogenesis in eel. Expression of cyclin A2 increased 1 day after the induction of spermatogenesis and reached a plateau after 6 days when many type B spermatogonia were found. In contrast, the expression of cyclin A1 mRNA was detected after 9 days coincident with the first appearance of spermatocytes. Cyclin A1 mRNA was localized in germ cells of all stages from primary spermatocytes to round spermatids, whereas cyclin A2 mRNA was specifically localized in spermatogonia, secondary spermatocytes, round spermatids, and testicular somatic cells, including Sertoli cells. Dmc1 protein was localized only in the earlier stages of primary spermatocytes; prior to this stage cyclin A1 mRNA was not detectable. These results suggest that cyclin A2, Dmc1, and cyclin A1 are expressed in spermatogenic cells sequentially before and during meiosis, indicating that the entry of spermatogonia into meiotic prophase can be distinguished by Dmc1 protein and cyclin A1 mRNA expression. VI. Endocrine regulation of oocyte growth and maturationTwo major steroidal mediators, estradiol-17β and 17α, 20β-dihydroxy-4-pregnen-3-one (17α, 20β-DP) are syn- thesized by ovarian follicles during oocyte growth and final maturation in response to FSH and LH, respectively. Two cell-type models in which the thecal layer provides precursor steroids to the granulosa layer, have been demonstrated for estradiol-17β and 17α, 20β-DP production. Ad4BP/SF-1 serves as a transcriptional regulator for ovarian cytochrome P450 aromatase (P450arom) which converts testosterone to estradiol-17β in granulosa cells during vitellogenesis. LH induces a distinct shift in steroidogenesis, i.e. from estradiol-17β to 17α, 20β-DP as well as the steroidogenic enzyme genes from P450arom to 20β-hydroxysteroid dehydrogenase (20β-HSD), in the granulosa layers of ovarian follicles prior to oocyte maturation. The triggering of the steroidogenic shift by LH is manifested through two molecular mechanisms, the first being the subjugation of the expression of Ad4BP/SF-1 vis a vis P450arom, and the second, the induction of over-expression of 20β-HSD probably via CREB. Unlike estradiol-17β (genomic action), 17α, 20β-DP binds to a novel, G-protein-coupled membrane receptor (non-genomic action) (Fig. 3), leading to the de novo synthesis of cyclin B, the regulatory component of maturation-promoting factor (MPF), which activates a preexisting 35-kDa cdc2 kinase via phosphorylation of its threonine 161 by a threonine kinase, thus producing the 34 kDa active cdc2. Upon egg activation, MPF is inactivated by degradation of cyclin B. We showed that the 26S proteasome initiates cyclin B degradation through the first cut of its NH2 terminus at lysine 57. |
 |
|
Fig. 3 Hormonal regulation of meiotic maturation in fish oocytes. Three major mediators, LH (pituitary), 17α,20β-DP (ovarian follicle) and MPF (oocyte), are involved. Both 17α,20β-DP and an endocrine-disrupting chemical, DES, may interact with a novel membrane progestin receptor. |
|
An endocrine-disrupting chemical, diethylstilbestrol (DES), a nonsteroidal estrogen, triggers oocyte maturation in fish. The morphology (the time course of the change in germinal vesicle breakdown) and an intracellular molecular event (the de novo synthesis of cyclin B) induced by DES were indistinguishable from those induced by 17α, 20β-DP. A synergistic action of DES on 17α, 20β-DP-induced oocyte maturation was observed. Both 17α, 20β-DP- and DES-induced oocyte maturation was inhibited by an antibody against the 17α, 20β-DP receptor. These results suggest that DES may act on the 17α, 20β-DP receptor as an agonist of 17α, 20β-DP (Fig. 3). |
Publication List:Horiguchi, R., Tokumoto, M., Nagahama, Y. and Tokumoto, T. (2005). Molecular cloning and expression of cDNA coding four spliced isoforms of casein kinase Iα in goldfish oocytes. Biochim. Biophys. Acta 1727, 75-80. Horiguchi, R., Yoshikuni, M., Tokumoto, M., Nagahama, Y. and Tokumoto, T. (2004). Identification of a protein kinase which phosphorylates a subunit of the 26S proteasome and changes in its activity during meiotic cell cycle in goldfish oocytes. Cell. Signal. 17, 205-215. Kita, M., Nakamura, Y., Okumura, Y., Ohdachi, S.D., Oba, Y., Yoshikuni, M., Kido H. and Uemura, D. (2004). Blarina toxin, a mammalian lethal nenom from the short-tailed shrew Blarina brevicauda: Isolation and characterization. Proc. Natl. Acad. Sci. USA 101, 7542-7547. Kobayashi-Kajiura, H., Kobayashi, T. and Nagahama, Y. (2004). The cloning of cyclin B3 and its gene expression during hormonally induced spermatogenesis in the teleost, Anguilla japonica. Biochem. Biophys. Res. Commun. 323, 288-292. Kobayashi-Kajiura, H., Kobayashi, T. and Nagahama, Y. (2004). Cloning of cDNAs and the differential expression of A-type cyclins and Dmc1 during spermatogenesis in the Japanese eel, a teleost fish. Dev. Dyn. (in press). Kobayashi, T., Kobayashi, H. and Nagahama, Y. (2004). Two DM domain genes, DMY and DMRT1, involved in testicular differentiation and development in the medaka, Oryzias latipes. Dev. Dyn. 231, 518-526. Kobayashi, Y., Kobayashi, T., Nakamura, M., Sunobe, T., Morrey, C.E., Suzuki, N. and Nagahama, Y. (2004). Characterization of two types of cytochrome P450 aromatase in the serial-sex changing gobiid fish, Trimma okinawae. Zool. Sci. 21, 417-425. Mita, M., Oka, H., Thorndyke, M.C., Shibata, Y., Yoshikuni, M. and Nagahama, Y. (2004). Inhibitory effect of a SALMFamide neuropeptide on secretion of gonad- stimulating substance from radial nerves in the starfish Asterina pectinifera. Zool. Sci. 21, 299-303. Nagahama, Y., Nakamura, M., Kitano, T. and Tokumoto, T. (2004). Sexual plasticity in fish: A possible target of endocrine disruptor action. Environ. Sci. 11,73-82. Senthilkumaran, B. and Nagahama, Y. (2004). A shift in steroidogenesis occurring in ovarian follicles prior to oocyte maturation. Mol. Cell. Endocrinol. 215, 11-18. Sunobe, T., Nakamura, M., Kobayashi, Y., Kobayashi, T. and Nagahama, Y. (2004). Gonadal structure and P450scc and 3β-HSD immunoreactivity in the gobiid fish Trimma okinawae during bi-directional sex change. Ichthyol. Res. 52 (in press). Suzuki, A., Tanama, M., Nagahama, Y. and Shibata, N. (2004). Expression of aromatase mRNA and effects of aromatase inhibitor during ovarian development in the medaka, Oryzias latipes. J. Exp. Zool. 301A, 266-273. Tokumoto, T., Tokumoto, M., Horiguchi, R., Ishikawa, K. and Nagahama, Y. (2004). Diethylstilbestrol induces fish oocyte maturation. Proc. Natl. Acad. Sci. USA 101, 3686-3690. Wakata, Y., Tokumoto, M., Horiguchi, R., Ishikawa, K., Nagahama, Y. and Tokumoto, T. (2004). Identification of a-type subunits of the Xenopus 20S proteasome and analysis of their changes during the meiotic cell cycle. BMC Biochem. 5, 18-25. Wang, D.S., Zhou, L.Y., Kobayashi, T. and Nagahama, Y. (2004). Molecular cloning and gene expression of Foxl2 in the Nile tilapia, Oreochromis niloticus. Biochem. Biophys. Res. Comm. 320,83-89. |
