| DIVISION OF DEVELOPMENTAL GENETICS | ||||||||
|
||||||||
1) Graduate School of Biological Sciences, University of Tsukuba |
||||||||
The sperm and egg, or the germ cells
are the specialized cells, which can transmit the genetic materials
from one generation to the next in sexual reproduction. All the other
cells of the body are somatic cells. This separation of germ and somatic
cells is one of the oldest problems in developmental biology. In many
animal groups, a specialized portion of egg cytoplasm, or germ plasm,
is inherited by the cell lineage which gives rise to germ cells. This
cell lineage is called germline. The germline progenitors eventually
migrate into the gonads, where they differentiate as germ cells when
the organisms are physically matured. Earlier investigators have demonstrated
that germ plasm contains maternal factors required and sufficient
for germline formation. In the fruit fly, Drosophila, this cytoplasm
is histologically marked by the presence of polar granules, which
act as a repository for the maternal factor required for germline
formation. Our molecular screens have identified several factors stored
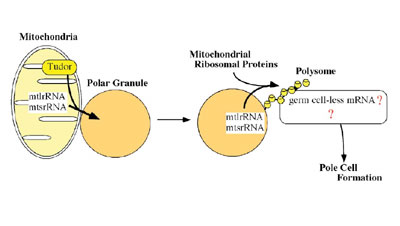
in the polar granules. One of the factors is mitochondrial large rRNA
which functions to form the germline progenitors, or pole cells. The
other is nanos mRNA, which is required for pole cell differentiation.
Ultrastructural studies have shown that the germ plasm is basically composed of polar granules and mitochondria. While the primary roles of the mitochondria are oxidative phosphorylation and biosynthesis of many metabolites, it has now become evident that they are also involved in germline formation. In Drosophila, pole cell formation requires the function of mitochondrial ribosomal RNA in germ plasm. We have previously reported that mitochondrial large rRNA (mtlrRNA) and small rRNA (mtsrRNA) are both transported from mitochondria to polar granules. This transportation occurs during early embryogenesis, when mitochondria are tightly associated with polar granules in germ plasm, and it depends on the function of the maternally-acting gene, tudor, that is known to be required for pole cell formation. Mitochondrial rRNAs remain on the polar granules until pole cell formation and are no longer discernible on the granules within pole cells. Reduction of the extra-mitochondrial mtlrRNA amount results in the failure to form pole cells and injection of mtlrRNA is able to induce pole cells in embryos whose ability to form these cells has been abolished by uv-irradiation. These observations clearly show that the extra-mitochondrial mtlrRNA on polar granules has an essential role in pole cell formation, presumably cooperating with mtsrRNA. Recently, we found that mitochondrial rRNAs form mitochondrial-type
of ribosomes on polar granules, cooperating with mitochondrial ribosomal
proteins. This suggests the possibility that the protein (s) essential
for pole cell formation is produced by the mitochondrial-type of ribosomes.
To address this issue, we examined the effect of Chloramphenicol and Kasugamycin on pole cell formation. Chloramphenicol and Kasugamycin are known to inhibit mitochondrial (prokaryotic)-type
of translation. When these antibiotics were injected into the posterior
pole region of early embryos, pole cell formation was severely affected.
In contrast, Chloramphenicol and Kasugamycin treatment
did not affect somatic cell formation at a dose we used. These observations
strongly suggest that the mitochondrial-type of translation system
must be intact for the embryos to form pole cells. The project to
identify a targer mRNA for the mitochondrial-type of translation machinery
is now on going.
Pole cells differ from the soma in regulation of mitosis and transcriptional activity. Pole cells cease mitosis at gastrulation and remain quiescent in the G2 phase of the cell cycle throughout their migration to the gonads, while somatic cells continue to proliferate during the rest of embryogenesis. Furthermore, pole cells are transcriptionally quiescent until the onset of gastrulation, although transcription is initiated in the soma during the syncytial blastoderm stage. Consistent with this, RNA polymerase II (RNAP II), but not RNA polymerase I, remains inactive in early pole cells. Thus, the ability to express zygotic mRNA-encoding genes is suppressed only in pole cells in early embryos. Among the maternal components of germ plasm, Nanos (Nos) is essential for the germline-specific events occurring in pole cells. nos mRNA is localized in the germ plasm during oogenesis, and is translated in situ to produce Nos protein after fertilization. Nos is only transiently present in the posterior half of embryos during the preblastoderm stage, and is required there for posterior somatic patterning. Nos in the germ plasm is more stably inherited into the pole cells at the blastoderm stage, remaining detectable in these cells throughout embryogenesis. Pole cells that lack Nos (nos- pole cells) are unable to follow normal germline development; they fail to migrate properly into the embryonic gonads, and consequently do not become functional germ cells. In nos- pole cells, mitotic arrest at G2 phase is impaired, and they undergo premature mitosis. Furthermore, nos- pole cells fail to establish and/or maintain transcriptional quiescence, and ectopically express somatically-transcribed genes, including fushi tarazu (ftz), even-skipped (eve) and Sex-lethal (Sxl). Nos represses translation of mRNAs with discrete RNA sequences called
Nos response elements (NREs). In the pathway leading to posterior
somatic patterning, Nos acts together with unlocalized Pumilio (Pum)
protein to repress translation of maternal hunchback (hb)
mRNA. This translational repression is mediated by binding of Pum
to NREs in the 3'-untranslated region (UTR) of hb mRNA. In pole cells,
Nos also acts with Pum to regulate germline-specific events. Pum,
like Nos, is required in pole cells for their migration to the gonads.
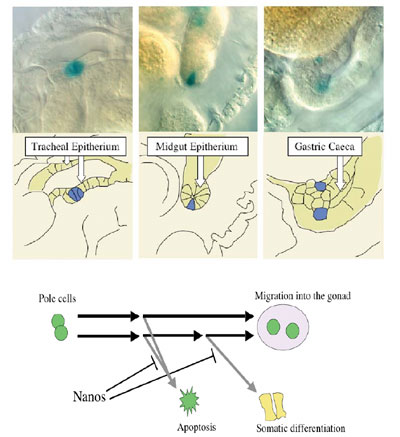
We found that Nos, along with Pum, represses translation of importin a2 (impa2) mRNA in early pole cells. The impa2 mRNA contains an NRE-like sequence in its 3'-UTR and encodes a Drosophila importin a homologue that plays a role in nuclear import of karyophilic proteins. We found that Nos inhibits expression of a somatically-transcribed gene, ftz, in pole cells by repressing Impa2-dependent nuclear import of a transcriptional activator for ftz, Ftz-F1. Furthermore, the expression of another somatic gene, eve, and RNA Polymerase II activity are also repressed by Nos in pole cells through its effects on Impa2-dependent nuclear import. The above results raise the question whether the pole cells lacking
Nos (nos- pole cells) are able to differentiate
into somatic cells. However, it is difficult to study their developmental
fate, since Nos also represses apoptosis of pole cells, and almost
all of nos- pole cells are eliminated until at
least the end of embryogenesis. To overcome this problem, we used
Df (3L) H99, a deletion for three genes required for apoptosis. Introduction
of the H99 deficiency results in nos- pole cells being escaped from
apoptosis. We transplanted the nos- H99- pole cells into normal embryos and observed their behavior, and found
that some of nos- H99- pole cells were
able to differentiate as somatic cells. This suggests that pole cells
have the ability to differentiate as somatic cells, but its ability
is inhibited by Nanos activity. Recently, we have found that somatic
differentiation of nos- H99- pole cells
requires Impa-2 activity, suggesting that
Nos inhibits somatic differentiation by repressing Impa-2
production.
Pole cells migrating into the gonads are specified to be the primordial
germ cells (PGCs). It has been believed that zygotic genes expressed
in pole cells within the gonads are required for their fate specification.
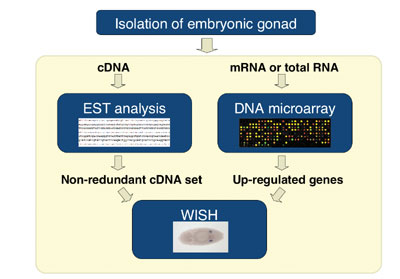
To explore the regulatory mechanism of germline specification, we
attempted to identify genes expressed in pole cells and/or in somatic
cells within the gonad by a comprehensive approach. From the embryos
carrying EGFP-vasa transgene that express GFP only in pole cells,
we isolated the gonads by using fluorescence-activated cell sorting
(FACS), and costructed a gonad cDNA library. Each cDNA clone was sequenced
from both 5’ and 3’ ends, and these Expression Sequence
Tags (ESTs) were computationally condensed into sequence clusters,
which were then subjected to whole-mount in situ hybridization
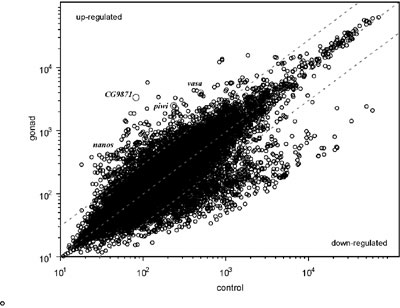
(WISH). Approximately 20,000 of ESTs were generated, and were clustered
into 2900 distinct genes. The WISH analysis identified more than 130
genes that were expressed predominantly in the gonads. In addition,
we found gonad-specific splicing form in some transcripts. These transcriptome
data will allow us to illustrate genetic networks governing the germline
specification.
It has been believed that maternal factors localized in germ plasm
may ultimately trigger germline-specific events, such as meiosis.
We have isolated an X-linked maternal mutation, sva53 that
affects meiosis. Pole cells that were formed in the embryos derived
from sva53 homozygous germline clone (sva53 pole
cells) were able to develop into the oocytes, but they failed to execute
meiosis. We also found that the germline-specific expression of vasa
gene was severely affected in sva53 pole cells. These results
suggest that the maternal factor encoded by sva53 gene may
activate gene expression, which is essential for meiosis. In order
to identify sva53 gene, we mapped sva53 mutation
to 200 kb-genomic region of 11C by using duplications and deficiencies.
Within this region, we found a gene encoding a Zn-finger transcription
factor, of which mRNA is maternally supplied into embryos. |