| DIVISION OF CELL DIFFERENTIATION | ||||||||
|
||||||||
| 1) CREST, JST Postdoctoral
Fellow 2) Graduate School of Kyushu University 3) Graduate University for Advanced Studies 4) Graduate School of Tohoku University 5) CREST, JST Technical Assistant 6) CREST, JST Secretary |
||||||||
Although sexual dimorphism manifests most obviously in the gonads (testis and ovary), it can be observed in the other parts of the entire animal body. For instance, it is well known that several tissues such as the external genitalia, muscle, and brain exhibit sexual dimorphisms in terms of their structures and functions. This process of sex differentiation can be divided into three steps. The first occurs at fertilization, during which the sexes of fertilized eggs are determined genetically by combination of sex chromosomes. As the second step, the individuals carrying XY and XX sex chromosomes develop the testis and ovary, respectively. Sex differentiation of the gonads usually proceeds during fetal stages. Thereafter, the gonads control the sexes of the whole body through the function of sex steroids synthesized in the sexually differentiated gonads. Therefore, the gonadal sexes are quite important for sex differentiation of the animals. It has been established that a number of transcription factors play
crucial roles in the process of gonadal differentiation. Some of these
factors, such as SRY, WT1, DAX-1, SOX9 and ARX were identified as
the products of genes responsible for human diseases that display
structural and functional defects in tissues including the gonads.
The functions of the other transcription factors such as Ad4BP/SF-1,
Emx2, M33, and Lhx9 were defined by the phenotypes of the gene disrupted
mice. In addition, the expression profiles with respect to their distribution
and sexual dimorphism strongly suggested the functional significance
at the early stage of gonadal differentiation. However, it remains
to be clarified how the transcription factors above regulate their
target gene transcription and how the genes encoding the transcription
factors are regulated by upstream regulators. Studies from above two
directions are quite important to define the gene regulatory cascade
and the molecular mechanisms that supports sex differentiation of
the gonad. It has been well documented that dioxins acts as an endocrine disruptor
through estrogenic action. However, the molecular mechanisms underlying
this action have been largely unknown. We have clarified it by focusing
on the reproductive defects displayed in the AhR gene disrupted mouse.
The gene disrupted female mice showed significantly reduced fertility
probably due to disordered estrus cycle, low concentration of ovarian
estradiol, and reduced numbers of ovulated eggs. Since estradiol is
a representative sex steroid of female mainly produced in the ovary
by successive reactions of steroidogenic enzymes, the expression of
these steroidogenic genes were investigated, and found that the expression
of cytochrome P450 aromatase catalyzing the final step of estrogen
synthesis is affected significantly in the gene disrupted mouse. Showing
a good correlation, estrogen treatment rescued the reduced ovulation
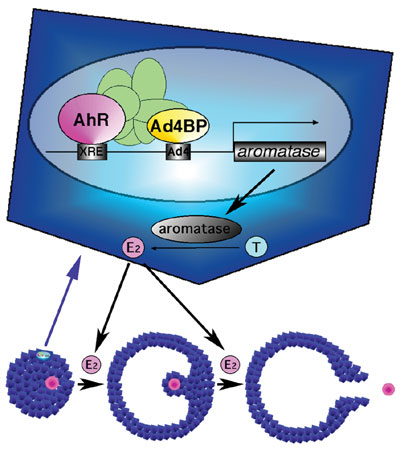
capacity. This remarkable phenotype suggested that AhR regulates the P450 aromatase gene transcription. Indeed, reporter gene
analysis indicated that the mouse and human P450 aromatase genes were activated by AhR.
Our previous study revealed that Ad4BP/SF-1, a member of nuclear receptor,
is expressed specifically in the steroidogenic tissues including the
ovary, and implicated in regulation of the steroidogenic genes. Therefore,
it was investigated whether AhR activates the P450 aromatase
gene transcription synergistically with Ad4BP/SF-1. Interestingly,
reporter gene assays showed synergy between the two factors. Showing
a good correlation, these two factors were indicated to interact mutually,
and moreover chromatin immunoprecipitation assays with anti-AhR and
anti-Ad4BP/SF-1 antibodies revealed that both factors bind their recognition
sequences on the aromatase promoter and formed a protein
complex in the ovarian cells. Thus, together with the AhR
KO phenotype, it was concluded that AhR regulates the P450 aromatase
gene transcription with Ad4BP/SF-1, and thereby regulates estrogen
synthesis in the ovary (Fig. 1). This finding clearly elucidated that
dioxins through binding to AhR exert estrogenic action by upregulating
P450 aromatase gene expression in the ovary. (This study
was performed as collaboration with Prof. Fujii-Kuriyama (Tsukuba
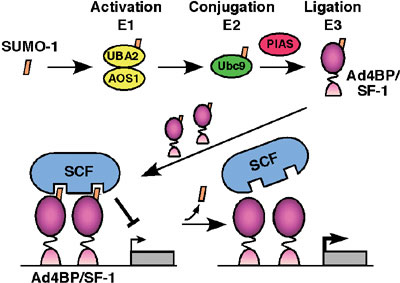
University).) In order to define the function of Ad4BP/SF-1, we have screened a two-hybrid library prepared form mouse embryonic gonads. UBC9, PIAS1 and PIAS3, all of which have been indicated as components for sumoylation reaction (conjugation of SUMO (small ubiquitin-like modifier)), were isolated as the interacting proteins. Thus, we investigated whether Ad4BP/SF-1 is sumoylated in vivo and in vitro. Immunoprecipitation study from cultured cells showed that at least a part of Ad4BP/SF-1 is sumoylated in cultured cells. In vitro reconstitution study using purified components revealed clearly that sumoylation occurs at two lysine residues, K119 and K194, of Ad4BP/SF-1. Moreover, interestingly, sumoylation at K194 is essential for the second sumoylation at K119, suggesting that conjugation of SUMO induces conformational alteration of Ad4BP/SF-1. With respect to the physiological function of SUMO, we have investigated
whether sumoylated Ad4BP/SF-1 differentially activates transcription,
since sumoylation consensus sequence is overlapped with that of synergy
control region originally defined as a suppression domain for synergistic
transcriptional activation. Ad4BP/SF-1 carrying mutation at the sumoylation
site enhanced synergistic transcription with reporter gene driven
by multiple Ad4 binding sites. It was established that Müllerian
inhibiting substance (MIS) gene is regulated by SOX9
synergistcally with Ad4BP/SF-1. Interestingly, SOX9 has a sumoylation
consensus sequence and in fact sumoylated as well. Therefore, we asked
whether sumoylation has effects on the synergy between the two transcription
factors. Expectedly, unsumoylated forms activated the synergistic
transcription of the MIS gene more than wild type. To define the molecular
mechanisms underlying the synergy enhancement, we asked with in
vitro sumoylated Ad4BP/SF-1 whether sumoylation affects DNA binding
activity and interaction with SOX9. However, sumoylation was found
to have no effect on both aspects, strongly suggesting the presence
of a putative synergy control factor (Fig. 2). Although the physiological
significance of sumoylation has been controversial, identification
of factors interacting preferentially with the sumoylated Ad4BP/SF-1
such as the synergy control factor will probably give us quite valuable
information. (This study has been performed as collaboration with
Profs. Kikuchi (Hiroshima Univ.), Shirakawa (Yokohama City Univ.),
and Yamanaka (Kyushu Univ.).)
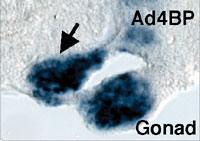
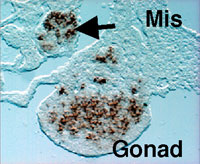
The gonad as well as the reproductive tracts, kidney, and adrenal cortex are derived from a part of the intermediate and lateral plate mesoderms. In addition, the mesonephros essential for the early gonadal development also originates from the intermediate mesoderm. Although the mesonephros was elucidated as the source of certain cell types constituting the testis, its contribution to gonad formation has been largely unknown. We examined in detail the expression profiles of Wnt4, Fgf8, and Fgf9 in the developing mesonephros of chick embryos, and found that the expressions of these factors are spatially and temporally correlated with those of the marker genes for the gonad and/or adrenal cortex such as Ad4BP/SF-1 and Dmrt-1 (doublesex- and mab-3-related transcription factor). As was shown previously with the rat fetuses, it was confirmed with the chick embryos that these two tissues are derived from a single cell population, the adreno-gonadal primordium. Thereafter, the primordium divided into two cell populations, one of which is the gonadal primordium and the other of which is the adrenal primordium. All of these cells are identified as immunoreactive cells for Ad4BP/SF-1. The expression of the growth factors, Wnt4, Fgf8, and Fgf9, are detectable in the nephroginous mesenchyme (feature mesonephros) and developing nephric tubules at the stage of the early gonad and adrenocortical development. Interestingly, the expressions of Wnt4 and Fgf9 are correlate with those of Ad4BP/SF-1 and Dmrt1. Therefore, it was assumed that these growth factors regulate the expression of the gonadal and adrenocortical marker gene expressions. In order to define it, Wnt4 and Fgf9 were misexpressed
with replication competent virus system in the gonad and adrenal cortex
of the early developmental stages. Misexpression of Wnt4 expanded the expression of Ad4BP/SF-1 as the marker for the
adrenogonadal primordium while failed to expand the expression of
Dmrt-1. Interestingly, misexpression of Fgf9 expanded the
expression of Dmrt-1 and probably thereby resulted in additional
gonad formation. In fact, the expressions of marker genes for the
gonad were observed even in the additional gonad (Fig. 3). Moreover,
chemical inhibitor for Fgf receptor downregulated the Dmrt-1 expression. Both gain-of-function and loss-of-function treatments
above induced no obvious structural alteration of the adrenal cortex,
indicating that Fgf9 induced gonad development probably through stimulating
cell proliferation or alternatively through trans-determining cell
fate into the gonad. This study demonstrated that Wnt4 and Fgf9 expressed in the developing mesonephros are involved
in the early stages of the gonad and adrenocortical development through
distinct functions.
In the case of mammals, a gene disruption study indicated that Fgf9 is essential for testicular differentiation. However, in the chick the misexpression of Fgf9 was unable to induce the expression of the testicular marker gene in the female gonad. This clear discrepancy might be due to the difference of experimental conditions, one of which is gene disruption (loss-of-function) study and the other is misexpression (gain-of-function) study, or due to difference of animal species. (This study has been performed as collaboration with Dr. Yoshioka in Hyogo Univ. of Teacher Education.) In addition to the studies described above, we have investigated tissue-specific enhancers localized in the Ad4BP/SF-1 gene. Recently, we identified a fetal adrenocortical-specific, pituitary-specific, and ventro-medial hypothalamic nucleus-specific enhancers, and their fine structures have been clarified. Investigation of the factors to bind the enhancers will be essential to clarify the mechanism underlying the tissue differentiation. It has been known that several gene disruption studies revealed their
implication into gonad differentiation and gonad sex differentiation.
However, functional and genetic correlation among the genes have remained
to be investigated. As the investigation of the genetic correlation,
analyses for genetic interaction have been performed with a certain
lines of gene disrupted mice, of which genetic backgrounds have been
fixed into C57B/6 and FVB in addition to the original 129J. Some of
the analyses already indicated the presence of the genetic interaction,
and the molecular basis for the interaction is under investigation. |
||||||||
| Publication List: Suzuki, T., Kasahara, M., Yoshioka, H., Morohashi, K., Umesono K (2003) LXXLL motifs in Dax-1 have target specificity for the orphan receptors Ad4BP/SF-1 and LRH-1. Mol. Cell. Biol. 23, 238-249. Meeks, J. J., Crawford S. E., T Russell, A., Morohashi, K, Capel, B., Weiss, J., and Jameson, J. L. (2003) Dax1 regulates testis cord organization during gonadal differentiation. Development 130, 1029-1036. Mizusaki, H., Kawabe, K., Mukai, T., Ariyoshi, E., Kasahara, M., Yoshioka, H., Swain, A., and Morohashi, K. (2003) Dax-1 gene transcription is regulated by Wnt4 in the female developing gonad. Mol. Endocrinol. 17, 507-519. Kawajiri, K., Ikuta, T., Suzuki, T., Kusaka, M., Watanabe, J., Muramatsu, M., Fujieda, K., Tachibana, M. and Morohashi, K. (2003) NR boxes of Dax-1 participate both in Ad4BP/SF-1 dependent nuclear import and in cytoplasmic retention of Dax-1. Mol. Endocrinol. 17, 994-1004. Kato, M., Das, S., Petras, K., Kitamura, K., Morohashi, K., Abuelo, D. N., Barr, M., Bonneau, D., Brady, A., Carpenter, N. J., Frisone, F., Fukuda, T., Guerrini, R., Iida, E., Itoh, M., Lewanda, A. F., Nanba, Y., Oka, A., Proud, V. K., Russel, K, L., Saugier-Veber, P., Schelley, S. L., Selicorni, A., Shaner, R., Silengo, M., Stewart, F., Sugiyama, N., Toyama, J., Toutain, A., Vargas, A. L, Yanazawa, M., Zackai, E. H. and Dobyns, W. B. Mutations of ARX are associated with striking pleiotropy and consistent genotype-phenotype correlation. Human Mutations in press Toda, K., Okada, Y., Zubair, M., Morohashi, K., Saibara, T., and Okada, T. Assessment of in vivo action of estrogen using aromatase-knockout mice which carry an estrogen-inducible enhanced green fluorescent protein gene. Endocrinol. in press. |