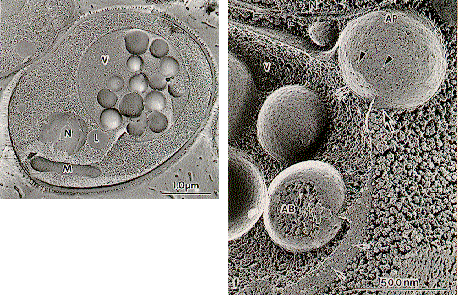
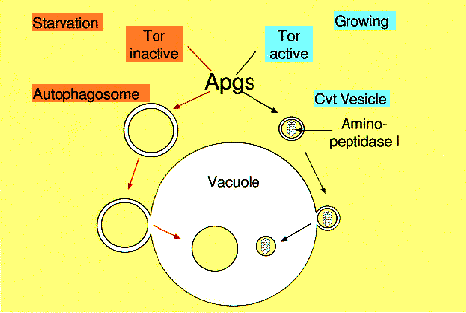
NATIONAL INSITUTE FOR BASIC BIOLOGY

National Institute for Basic Biology
DIVISION OF BIOENERGETICS
- Professor:
- Yoshinori Ohsumi
- Associate Professor:
- Tamotsu Yoshimori
- Research Associate:
- Takeshi Noda
- Yoshiaki Kamada
- Post Doctral Fellow:
- Tomoko Funakoshi1
- Noboru Mizushima2
- Graduate Students:
- Satoshi Kametaka (Univ. of Tokyo)
- Takayoshi Kirisako (Univ. of Tokyo)
- Technical Staff:
- Yukiko Kabeya
- Visiting Fellow:
- Fumi Yamagata (Teikyo Univ. of Sci. and Tech.)