NATIONAL INSITUTE FOR BASIC BIOLOGY

National Institute for Basic Biology
Division of Cellular Communication
(Adjunct)
- Professor:
- Ritsu Kamiya
- Associate Professor:
- to be appointed
- Research Associate:
- to be appointed
NATIONAL INSITUTE FOR BASIC BIOLOGY

National Institute for Basic Biology
|
|
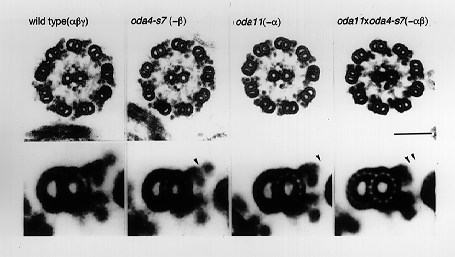
Fig. 1 Wild-type and mutant axonemes of Chlamydomonas that lack different heavy chains of outer arm dynein. Wild-type outer arm contains three heavy chains, a, b and g, of which the mutant oda4-s7 lacks b and oda11 lacks a. Lower photographs are the averaged images of the outer doublet microtubules, showing the structural defect in each mutant (arrowhead). |




nibb-adm@nibb.ac.jp
Last Modified: 12:00, June 27, 1997