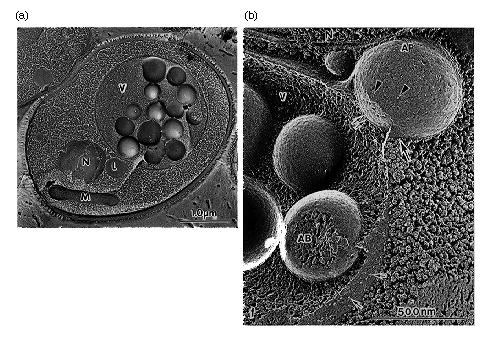
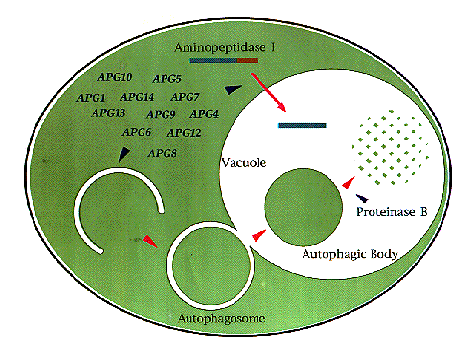
NATIONAL INSITUTE FOR BASIC BIOLOGY

National Institute for Basic Biology
Division of Bioenergetics
- Professor:
- Yoshinori Ohsumi
- Associate Professor:
- Tamotsu Yoshimori
- Research Associates:
- Takeshi Noda
- Yoshiaki Kamada
- Graduate Students:
- Kanae Shirahama (Univ. of Tokyo)
- Tomoko Funakoshi (Univ. of Tokyo)
- Satoshi Kametaka (Univ. of Tokyo)
- Chikara Tokunaga (Univ. of Tokyo)
- Takayoshi Kirisako (Univ. of Tokyo)
- Tomoko Funakoshi (Univ. of Tokyo)
- Technical Staff:
- Yukiko Kabeya
- Visiting Professor:
- Michael Filosa